The Fundamentals of Batteries: What Is Battery

Batteries are electrochemical devices that store chemical energy and convert it into electrical energy, powering our devices and facilitating our modern lives. They work by harnessing the movement of charged particles, called ions, between two electrodes, creating an electric current.
Electrochemical Reactions and Energy Storage
Batteries store energy through chemical reactions occurring within their components. The key elements are the electrodes, the electrolyte, and the separator.
- The electrodes, typically made of metals or conductive materials, serve as the sites where chemical reactions occur.
- The electrolyte, a liquid or paste, facilitates the movement of ions between the electrodes.
- The separator, a porous membrane, prevents the electrodes from directly contacting each other, ensuring a controlled flow of ions.
During discharge, the chemical reaction releases electrons from one electrode (anode) to the other (cathode), generating an electric current. The flow of electrons continues until the chemical reaction reaches equilibrium, signifying the battery’s depletion.
The chemical reaction in a battery is reversible, allowing for the storage and release of energy.
Battery Components and Their Functions
- Electrodes: These are the heart of the battery, where chemical reactions take place. The anode is the negative electrode, where oxidation occurs, releasing electrons. The cathode is the positive electrode, where reduction occurs, accepting electrons.
- Electrolyte: The electrolyte is a crucial component that allows ions to move between the electrodes, facilitating the flow of electric current. Different electrolytes are used in various battery types, depending on their properties and intended applications.
- Separator: This thin, porous membrane prevents the electrodes from coming into direct contact, preventing short circuits and ensuring a controlled flow of ions. The separator is essential for maintaining the battery’s safety and functionality.
- Case: The battery case provides structural support and protection for the internal components, ensuring their proper operation and preventing leakage.
- Terminals: These are the points of contact for external circuits, allowing the battery to be connected to devices and systems.
Analogy: A Water Pump, What is battery
Imagine a water pump with two tanks, one higher than the other. The higher tank represents the cathode, the lower tank represents the anode, and the pump represents the chemical reaction within the battery.
- The pump uses energy to move water from the lower tank to the higher tank, creating a pressure difference.
- When the pump is turned off, the water flows back from the higher tank to the lower tank, releasing energy.
This analogy illustrates how batteries store and release energy through chemical reactions, creating a potential difference between the electrodes, similar to the pressure difference in the water tanks.
Types of Batteries
- Lead-acid batteries: These are commonly used in vehicles, providing high current for starting the engine. They are relatively inexpensive and durable but have a lower energy density compared to other types.
- Lithium-ion batteries: These are widely used in portable electronics, electric vehicles, and grid-scale energy storage. They offer high energy density, long lifespan, and low self-discharge rates.
- Alkaline batteries: These are commonly used in everyday devices like flashlights and remote controls. They provide a good balance of energy density, cost, and shelf life.
- Nickel-cadmium (NiCd) batteries: These are known for their high durability and ability to withstand extreme temperatures. They are commonly used in power tools and emergency lighting systems.
- Nickel-metal hydride (NiMH) batteries: These offer higher energy density and lower environmental impact than NiCd batteries. They are often used in hybrid vehicles and portable electronics.
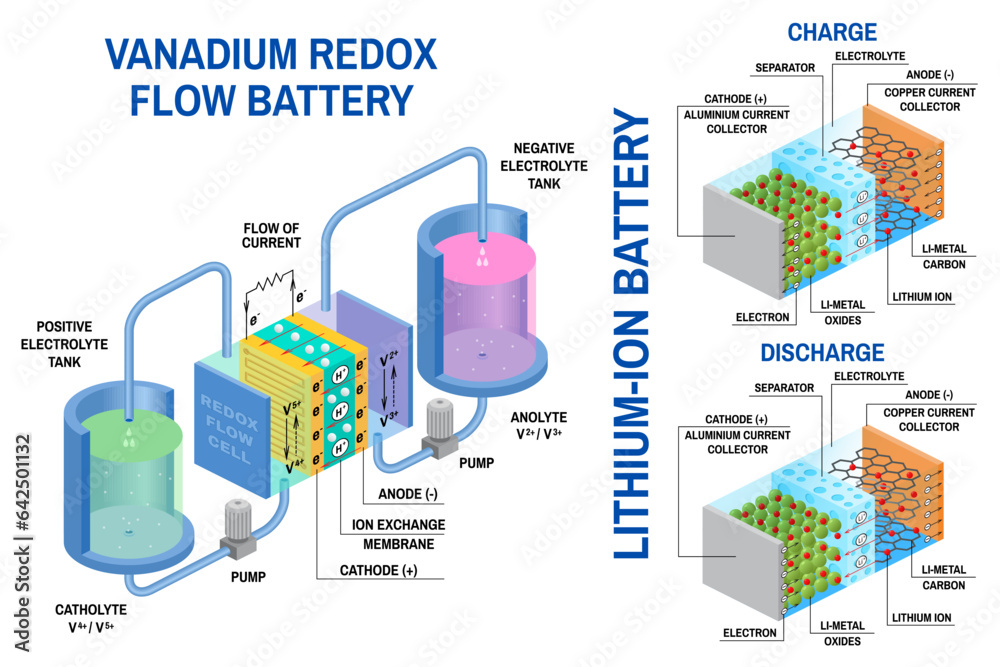
Battery Chemistry and Reactions

The heart of a battery lies in its intricate chemical reactions, a delicate dance of electrons and ions that transforms chemical energy into electrical energy. Understanding these reactions is key to comprehending how batteries store and release energy, their limitations, and the factors influencing their performance.
Electrochemical Reactions
The foundation of battery operation rests upon electrochemical reactions, where chemical energy is converted into electrical energy and vice versa. These reactions occur at the interface between the electrodes and the electrolyte, a conductive medium facilitating ion movement. During discharge, the chemical reactions release electrons, generating an electrical current. Conversely, during charging, an external electrical current drives the reverse reaction, storing chemical energy within the battery.
The Role of Electrodes and Electrolytes
Electrodes, the conductive materials within a battery, play a crucial role in facilitating the chemical reactions. They serve as the sites where oxidation and reduction reactions occur. The anode, the negative electrode, undergoes oxidation, releasing electrons and forming positively charged ions. The cathode, the positive electrode, undergoes reduction, accepting electrons and forming negatively charged ions. The electrolyte, a liquid or solid solution, acts as a conductor for ions, allowing them to migrate between the electrodes, completing the circuit and facilitating the flow of electricity.
Factors Influencing Battery Capacity and Lifespan
Several factors influence the capacity and lifespan of a battery, including:
- Electrode Materials: The choice of electrode materials significantly impacts battery performance. Materials with high capacity and good conductivity contribute to a higher energy density and longer lifespan.
- Electrolyte Properties: The electrolyte’s conductivity, stability, and reactivity with the electrodes influence the battery’s performance. Electrolytes with high conductivity enable faster ion transport, while stability ensures a longer lifespan.
- Battery Design: The physical design of the battery, including the electrode geometry and separator thickness, affects its capacity, power output, and safety.
- Temperature: Temperature extremes can negatively affect battery performance. High temperatures accelerate chemical reactions, potentially leading to degradation and reduced lifespan. Conversely, low temperatures can slow down reactions, reducing power output.
- Charging and Discharging Rates: Rapid charging and discharging can stress the battery, leading to faster degradation. Optimal charging and discharging rates vary depending on the battery chemistry and design.
- Depth of Discharge: Deep discharges, where the battery is drained to a low state of charge, can accelerate degradation. Minimizing deep discharges can prolong the battery’s lifespan.
Comparing Battery Chemistries
Different battery chemistries exhibit distinct chemical reactions and performance characteristics. Here’s a comparison of some common battery types:
| Battery Type | Anode | Cathode | Electrolyte | Chemical Reaction | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Lead-Acid | Lead | Lead Oxide | Sulfuric Acid | Pb + PbO2 + 2H2SO4 ↔ 2PbSO4 + 2H2O | Low cost, high capacity | Heavy, bulky, short lifespan |
| Lithium-Ion | Graphite | Lithium Cobalt Oxide | Lithium Salt in Organic Solvent | LiC6 + LiCoO2 ↔ 6C + Li1-xCoO2 + Lix+ + xe– | High energy density, long lifespan | Expensive, safety concerns |
| Nickel-Cadmium | Cadmium | Nickel Oxide Hydroxide | Potassium Hydroxide | Cd + 2NiO(OH) + 2H2O ↔ Cd(OH)2 + 2Ni(OH)2 | High power output, long lifespan | Toxic cadmium, lower energy density |
Battery Applications and Use Cases
Batteries, as the energy storage units, have become indispensable across various industries, revolutionizing the way we power our lives. Their versatility and adaptability have led to a wide range of applications, impacting everything from our daily commutes to the exploration of space.
Battery Applications Across Industries
Batteries have become ubiquitous, finding their way into countless applications across various industries. The following table provides a glimpse into the diverse applications of batteries in different sectors:
| Industry | Application | Example |
|---|---|---|
| Consumer Electronics | Powering mobile devices, laptops, tablets, smartwatches, and headphones. | Smartphones, laptops, wearables. |
| Automotive | Electric vehicles (EVs), hybrid electric vehicles (HEVs), and plug-in hybrid electric vehicles (PHEVs). | Tesla Model S, Toyota Prius, Chevrolet Volt. |
| Energy Storage | Grid-scale energy storage, residential energy storage, and off-grid power systems. | Large-scale battery farms for grid stabilization, home battery systems for backup power, solar-powered homes and communities. |
| Medical Devices | Pacemakers, defibrillators, insulin pumps, hearing aids, and other implantable devices. | Cardiac pacemakers, implantable defibrillators, insulin pumps. |
| Aerospace | Powering satellites, spacecraft, and drones. | SpaceX Falcon 9 rocket, NASA Mars rovers, commercial drones for delivery and surveillance. |
Advantages and Disadvantages of Battery Use
The use of batteries offers numerous advantages, but it also comes with certain limitations.
Advantages
- Clean Energy Source: Batteries are a clean energy source, as they do not produce emissions during operation, contributing to a cleaner environment.
- Portable and Flexible: Batteries are portable and flexible, allowing for power to be taken anywhere and used in various applications.
- Reliable and Efficient: Batteries offer high energy density and efficiency, providing reliable and consistent power output.
- Long Lifespan: With proper maintenance, batteries can have a long lifespan, reducing the need for frequent replacements.
Disadvantages
- Cost: Batteries can be expensive, especially for large-scale applications, posing a barrier to widespread adoption.
- Limited Capacity: Batteries have a limited capacity, meaning they can only store a certain amount of energy before needing to be recharged.
- Safety Concerns: Battery safety is a concern, as they can overheat or catch fire under certain conditions.
- Environmental Impact: Battery production and disposal can have environmental impacts, requiring responsible recycling and disposal practices.
Challenges and Opportunities for Battery Technology
Battery technology is constantly evolving, facing challenges and presenting opportunities for innovation.
Challenges
- Improving Battery Life and Capacity: Extending battery life and increasing energy storage capacity remain key challenges in the pursuit of longer-lasting and more powerful batteries.
- Reducing Cost: Lowering the cost of battery production is essential for making batteries more accessible and affordable for a wider range of applications.
- Addressing Safety Concerns: Ensuring the safety of batteries is crucial, requiring robust safety measures and regulations to prevent incidents like overheating or fires.
- Sustainable Battery Production and Disposal: Developing sustainable battery production processes and recycling solutions is vital for minimizing environmental impact.
Opportunities
- Advancements in Battery Chemistry: Research and development in battery chemistry are leading to new materials and technologies that promise higher energy density, faster charging times, and improved safety.
- Solid-State Batteries: Solid-state batteries offer the potential for higher energy density, faster charging, and improved safety compared to traditional lithium-ion batteries.
- Integration with Renewable Energy: Batteries are crucial for integrating renewable energy sources like solar and wind power, enabling a more sustainable energy future.
- Emerging Applications: Batteries are finding their way into new and exciting applications, such as electric aviation, grid-scale energy storage, and medical devices.
Energy Flow in a Battery-Powered Device
[Insert a diagram or flowchart illustrating the energy flow in a battery-powered device. Describe the diagram in detail, including the components and their roles in the energy flow.]
What is battery – Battery, in the legal context, refers to an intentional act that causes harmful or offensive contact with another person. This can be a physical assault, but it can also involve actions like spitting or throwing objects at someone. A recent example of this in the news was the arrest of actress Skai Jackson, skai jackson arrested , which involved allegations of battery.
The definition of battery can vary slightly depending on the jurisdiction, but the underlying principle remains the same: it involves an unwanted and harmful or offensive physical contact.
In basic terms, a battery is a device that stores chemical energy and converts it into electrical energy. This energy storage is crucial for powering many devices, from our smartphones to electric vehicles. However, the term “battery” also has a darker meaning in the context of interpersonal violence, specifically in the legal term domestic battery , which refers to the unlawful use of force against another person within a domestic relationship.
While these two meanings of “battery” are vastly different, they both highlight the potential for both positive and negative outcomes associated with the term.